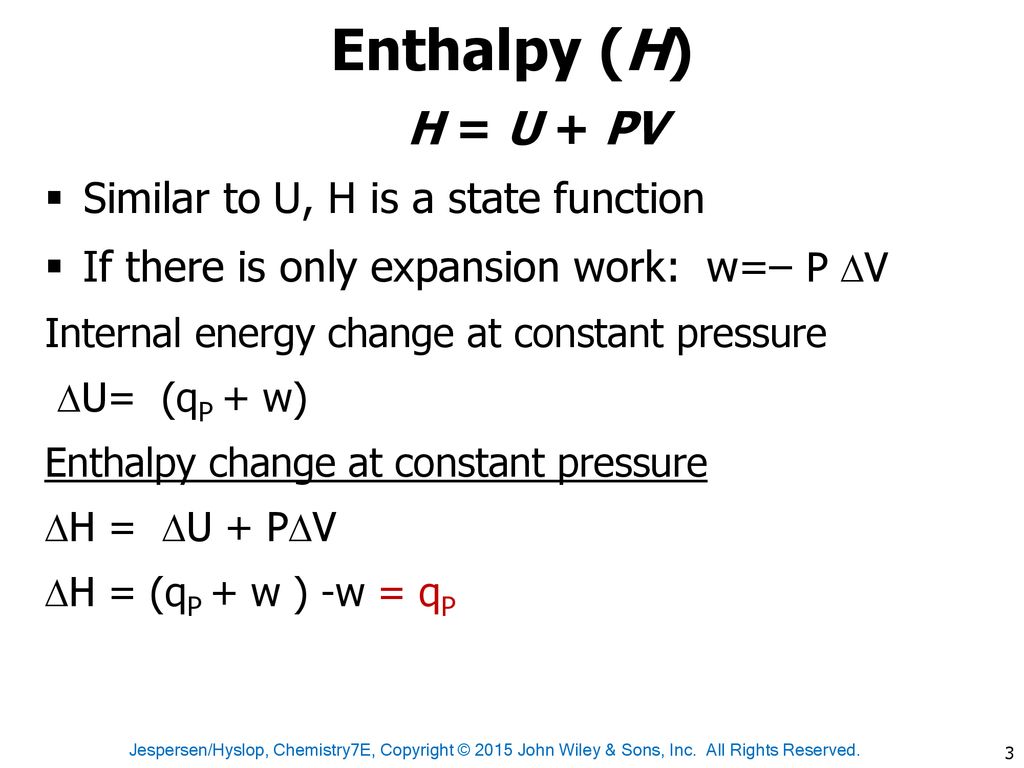
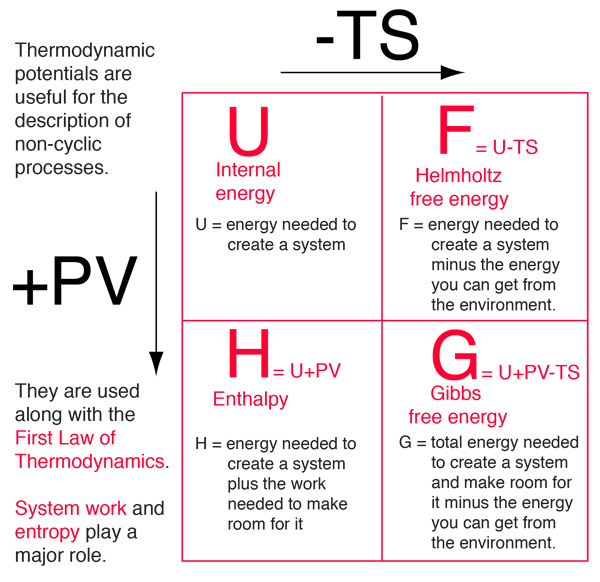
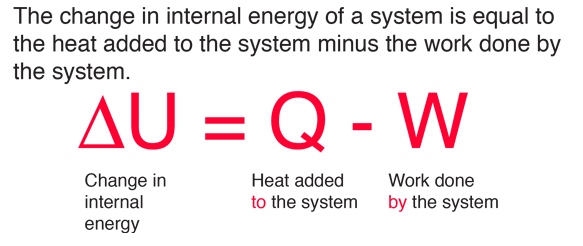
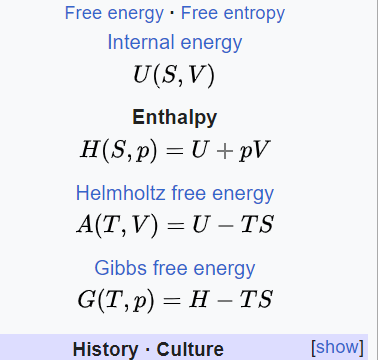The internal energy of an ideal gas is sum of totalkinetic energy of all molecules. Consider an idealgas in which the relation among U, P and V isU =2 +3 PV. The

Slide 1 of The First Law of Thermodynamics Internal Energy, U. Total energy (potential and kinetic) in a system. Translational kinetic energy. - ppt download

Thermodynamic Properties Property Table w Property Table -- from direct measurement w Equation of State w Equation of State -- any equations that relates. - ppt download
Derive the relation between ∆H and ∆U for an ideal gas. Explain each term involved in the equation. - Sarthaks eConnect | Largest Online Education Community

Difference Between Enthalpy and Internal Energy | Definition, Units, Formula for Calculation, Properties, Examples